
제약·의료기기 업종의 고객사들은 문서중앙화를 통해 보안과 협업 문제를 어떻게 해결했을까요?
업종 특성상 연구가 진행되는 곳이 많기에 문서 유실, 유출에 있어 매우 민감하며 대용량 데이터 보유량이 많아 쉽고 편리한 문서 공유와 체계적인 관리가 필요한 곳이죠.
실제 고객의 인터뷰를 통해, 사례를 확인해 보세요!
하이로닉
도입 연도: 2016년 / cloudium

하이로닉은 2016년에 클라우디움을 도입하셨습니다! 클라우디움 도입 전 하이로닉은 기존 NAS 서버를 사용하고 있었는데 회사에서 생성되는 문서 전체를 관리하기 쉽지 않았고 퇴직자의 PC 관리도 어려웠다 합니다.
NAS 서버의 경우는 접근 권한이 있는 사람이 문서를 사용한다면 언제, 누구에게로 반출되는지 확인할 방법이 없다는 점이 큰 단점이 있었는데요. 문서중앙화는 문서를 반출할 때 부서 문서 관리자의 승인을 얻어야 문서를 보낼 수 있는 외부 반출 프로세스로 문서 유출 사고를 막을 수 있죠! 게다가 문서를 보낼 때 파일을 그대로 전달하는 것이 아니라 URL을 통해 공유하여 관리자들은 중요 문서들의 흐름을 파악할 수 있게 되고, 외부 반출 문서가 언제, 어디서 다운받아졌는지 관리자가 확인할 수 있습니다.
일반적으로 퇴사자의 업무를 인수인계할 때, ①인계자가 자율적으로 자료를 백업하고 인수자에게 전달 + ②관리자는 해당 내용을 체크하고 퇴사자의 PC를 포맷하는 과정을 거칩니다. 이 과정에서 퇴사자가 악의적으로 자료를 삭제하거나 중요 문서가 있는지 모르고 이를 넘기지 않은 상태에서 PC가 포맷되어 유실될 우려가 있는데요.
문서중앙화 솔루션은 사용자가 서버에 저장된 문서를 삭제하더라도 언제든 휴지통에서 문서를 복구할 수 있습니다. 문서를 완벽하게 삭제하는 것은 관리자만 가능하죠! 그리고 인수인계를 사용자가 일일이 문서를 확인해 백업을 하는 방식으로 진행하는 것이 아니라 인수자의 권한을 변경함으로써 누락되는 문서 없이 쉽고 빠르게 진행할 수 있습니다.
경보제약
도입연도: 2019년 / Destiny ECM

경보제약은 타사의 문서중앙화 솔루션을 2012년부터 사용했던 기업입니다. 하지만 사용할수록 속도가 저하되고 오류가 너무 자주 발생하여 2019년에 사이버다임 Destiny ECM으로 시스템을 교체하게 되었죠. 기존 시스템을 교체하는 것이다 보니 업체 선정에 심혈을 기울여 속도, 안정성, 기술력 등 많은 요소를 꼼꼼히 비교한 결과, 수많은 레퍼런스와 뛰어난 기술력을 보유한 사이버다임 문서중앙화 솔루션이 선택되었습니다.
경보제약은 제약회사이다 보니 많은 연구 데이터 즉, 실험 자료들이 생성됩니다. 이 자료들의 보안을 강화하면서 체계적으로 관리할 수 있는 방법이 없을까 고민이라 하셨는데요. 이 고민은 Destiny ECM에 있는 프로젝트 그룹 관리 기능 해결! 실험 노트나 관련 문서들을 해당 그룹만 볼 수 있도록 권한 설정하여 철저하게 연구 개발 보안을 관리하게 되었습니다. 그리고 실험 자료들을 외부로 반출할 때는 임원진의 최종 결재를 받아야 가능하도록 설정해 더욱 강력히 외부 반출 문서를 관리할 수 있게 되었죠!
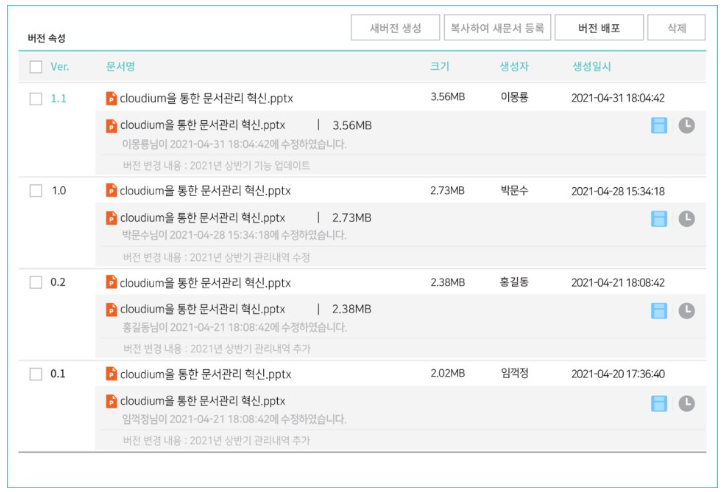
그리고 경보제약이 만족하는 또 하나의 강점! 바로 버전 관리 기능입니다. 기존에 사용하던 시스템은 폴더 단위 버전만 기록되고 문서는 별도로 버전 관리가 되지 않았다고 합니다. 그래서 일일이 버전을 넘버링 하여 수정하는 번거로움이 있었는데요. 하지만 사이버다임 문서중앙화 솔루션는 문서 내용 수정 후 문서를 닫으면 알아서 버전 관리 가능! 최신 문서를 찾아 헤매는 비효율을 줄일 뿐만 아니라 중복문서를 가지고 있을 필요가 없어져 효율적인 용량 사용도 가능해집니다^__^
바이젠셀
도입연도: 2021년 / cloudium

면역세포치료제 전문 기업 바이젠셀은 2021년부터 문서중앙화 솔루션 "클라우디움"을 사용하기 시작한 곳입니다. 문서중앙화 솔루션을 도입하기 전엔 업무 문서를 중앙 관리하고자 일일이 문서를 그룹웨어에 업로드해 해야 하는 불편함이 있었고, 다양한 사람이 문서를 이용하는 만큼 실수로 발생하는 문서 내용 수정 삭제로 문서 유실 방지가 필요했죠. 이런 문제점들이 어떻게 해결되었을까요?
바이젠셀은 약학 연구 개발 업종답게 대량의 실험 결과 데이터가 업무 산출물로 생성됩니다. 대용량 데이터를 USB에 담아서 개인 PC로 옮기려다 보니 느린 전송 속도로 인해 업무 효율이 떨어지고 데이터가 유실될 우려도 있었죠. 하지만 업무 산출물을 중앙 통합 저장소에 자동으로 저장하는 문서중앙화 솔루션이 도입되면서 번거롭게 USB로 옮길 필요도 없고, 문서를 일일이 그룹웨어에 업로드할 필요도 없어졌습니다! 게다가 모든 산출물들이 중앙 서버에 저장되어 있으니 재택근무를 진행하더라도 사무실에서와 큰 차이 없이 업무 연속성을 이어갈 수 있죠.
IT 전문 인력이 없음에도 문서중앙화 솔루션이 업무 플랫폼으로 잘 정착될 수 있었던 것은 클라우디움 시스템 자체의 손쉬운 권한 관리 설정 덕분이라고 말씀하셨는데요. 여기에 사용 가이드와 회사 내부 교육으로 빠르게 전 직원이 적응할 수 있었다고 합니다.
IT 인력 없이도 사이버 보안을 강화할 수 있는 문서중앙화 솔루션의 또 다른 특징들이 궁금하시다면?
중소기업의 고민, IT 인력 없이도 핵심 기술 유출 안전하게 막는 방법? 사이버다임 문서중앙화 솔루션!
PCL
도입연도: 2020년 / cloudium

체외 진단 의료기기 전문 기업인 PCL은 코로나19로 인해 진단키트 수요 증가로 기업이 성장하면서 체계적인 문서 관리와 중요 문서 보안의 필요성을 느껴 2020년에 클라우디움을 도입한 고객사입니다. 그리고 메신저와 이메일로 문서를 공유해오던 상황이라 본사와 지사 간의 문서 공유가 보다 수월해졌으면 했죠.
문서중앙화 솔루션의 장점! 바로 중앙 통합 저장소에 모든 문서를 저장하니 서버에 접근할 수 있는 환경이라면 언제 어디서든 문서를 확인할 수 있다는 점입니다. 즉, 본사에서 생성된 문서를 중앙 서버에 등록하면 동일한 중앙 서버를 사용하는 지사에서도 해당 문서를 바로 확인할 수 있다는 뜻이죠! 메일이나 메신저로 번거롭게 문서를 주고받을 필요 없이 즉각적으로 문서 활용이 가능하니 업무 효율성이 UP! UP!
또한, 퇴사자가 악의적인 마음으로 본인이 작성한 문서들을 삭제하고 퇴사하는 등 유실 사고도 미연에 방지하고, 문서중앙화 솔루션 속 휴지통 기능으로 삭제된 문서들을 찾아내고 복구하여 문서 유실 방지가 가능하니 문서의 내외부 유출은 물론, 문서 유실까지 막을 수 있죠!

 한국어
한국어








